RCT results are currently only published for the Zephyr and Spiration valves and are therefore included in this review. They are not the same.
 Pulmonx S Zephyr Data Bring Nice Thumbs Up For Endobronchial Valves Medtech Insight
Pulmonx S Zephyr Data Bring Nice Thumbs Up For Endobronchial Valves Medtech Insight
Hyperinflation occurs when air is trapped in the lungs and causes them to overinflate which is common for patients with emphysema.
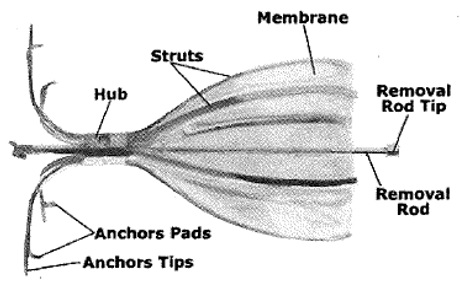
Spiration valve vs zephyr. Two different valve designs were tested in studies for bronchoscopic LVRS. The Spiration Valve is the second type of one-way valve approved by the U. National Institute for health and care Excellence new guidelines regarding bronchoscopic valves.
If so has it helped your breathing. The Zephyr valve system PulmonX Redwood City CA and the Spiration valve system Olympus Center Valley PA. If you have severe emphysema and are limited by shortness of breathing with usual daily activities you should consider being evaluated for possible placement of the these valves into.
This allows for bronchoscopic placement in selected airway regions and limits airflow to distal portions of the lung affected by emphysema. Appropriate patient selection is critical to the success of Zephyr Valve treatment. Has anyone had the valves put in.
Spiration valves are if the lung damage is primarily in the upper lobes of the lungs. The Zephyr valve procedure isnt designed to help all emphysema patients. The Zephyr EBV Pulmonx and the Spiration valve Olympus Corporation of the Americas.
The Spiration Valve System SVS. They were studied nearly simultaneously and the Principal Investigators were the same on both clinical trials. Also you must have hyperinflation of the lungs to be considered for this procedure.
The highlighted evaluation factors below are some of. FDA in 2018 for treatment of severe emphysema. Potential candidates should be referred to an experienced center for consideration of BLVR with EBV.
Formerly known as the intrabronchial valve consists of a one-way valve that blocks inspired airflow using a flexible umbrella design. Zephyr Valve Procedure Pros. Get a referral from your pulmonary to any hospital that will give you the extensive evaluation.
The German patient database study reported up to a three-year follow-up on 256 patients who had had an absence of collateral ventilation confirmed prior to receiving either the Zephyr or Spiration valves. Over time clinical trial data have helped define the successful patient profile. The selection criteria are based on clinical experience gathered to date on bronchial valve therapy and on peer-reviewed studies with the Spiration Valve System1 or the Zephyr Endobronchial Valve 278 in similar emphysematous patient populations.
1-467 The Zephyr Valve is the first endobronchial valve to receive approval from the FDA for patients with either heterogeneous and homogeneous emphysema and can be used to treat the upper and lower lobes of either lung. Its reserved for patients with a forced expiratory volume of less than 15. At present there are 2 FDA options for BLVR Zephyr Endobronchial Valve and Spiration Valve System that are indicated for patients with complete or near complete fissure integrity without collateral ventilation between contiguous lobes.
Spiration valve system produced by Olympus has now received FDA approval. An expert panel recommendation just published in Respiration named the Zephyr Endobronchial Valve from Pulmonx as the only minimally-invasive emphysema treatment to be used outside a clinical trial. Interventional Pulmonologist Gerard Criner MD FACP FACCP discusses the clinical benefits and patient selection requirements of bronchoscopic lung volume.
Zephyr valves are more for damage that is spread out over the entire lungs. Little information can be found in literature about the MedLung EBV and Miyazawa valve and whether these are currently used or not and therefore these valves are not included in this review. The Spiration valve was just approved early December by the FDA.
Has anyone on this forum had the Zephyr valves installed. Unlike lung volume reduction surgery the procedure is performed unilaterally due to the inherent procedural risk of pneumothorax. 37 At six months data available for 200 patients 37 of patients met the efficacy threshold for improvement in FEV 1 and both the six-minute walk and Modified Medical Research Council dyspnea.
Unfortunately initial bronchoscopic LVRS trials utilizing the Spiration valves and the Zephyr EBVs did not meet predetermined study endpoints and failed to obtain United States Food and Drug Administration FDA approval. There are currently 2 valve therapy options approved in the United States. In June the Zephyr Valve was approved for severe emphysema.
My understanding is that both the Zephyr and Spiration valves will. The SVS is similar to the Zephyr. The SVS has been.
 Olympus Launches Spiration Valve System For The Endobronchial Treatment Of Severe Emphysema Rtsleepworld
Olympus Launches Spiration Valve System For The Endobronchial Treatment Of Severe Emphysema Rtsleepworld
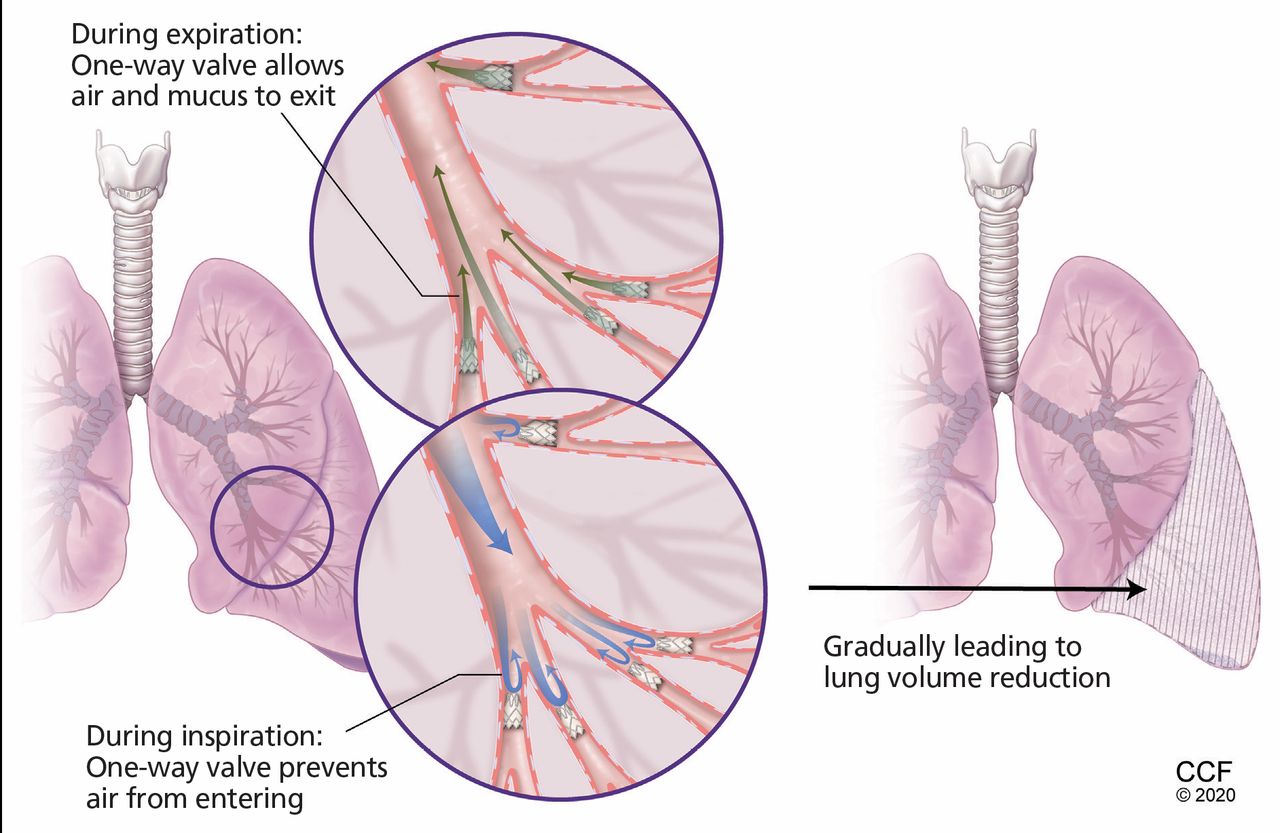 Bronchoscopic Lung Volume Reduction With Valves What Should The Internist Know Cleveland Clinic Journal Of Medicine
Bronchoscopic Lung Volume Reduction With Valves What Should The Internist Know Cleveland Clinic Journal Of Medicine
 Figure 4 From Phenotyping Copd Emphysema Semantic Scholar
Figure 4 From Phenotyping Copd Emphysema Semantic Scholar
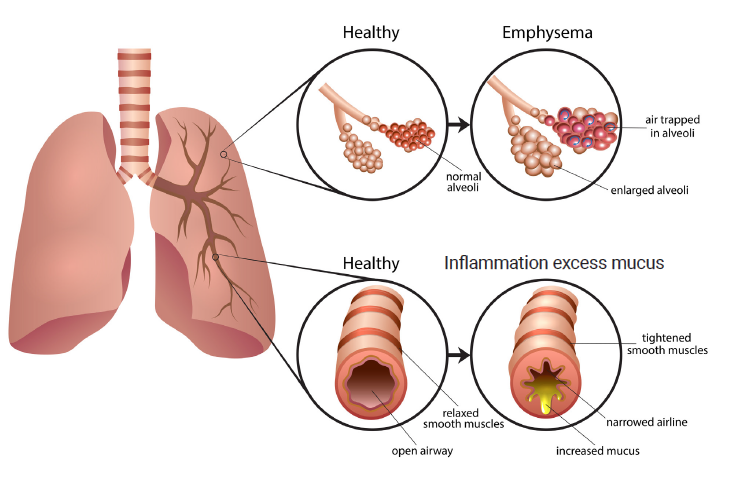 Endobronchial Valves For The Treatment Of Severe Emphysema Cadth Ca
Endobronchial Valves For The Treatment Of Severe Emphysema Cadth Ca
 Zephyr Endobronchial Valve Insitu Download Scientific Diagram
Zephyr Endobronchial Valve Insitu Download Scientific Diagram
 Bronchoscopic Lung Volume Reduction Status Quo Zantah Annals Of Translational Medicine
Bronchoscopic Lung Volume Reduction Status Quo Zantah Annals Of Translational Medicine
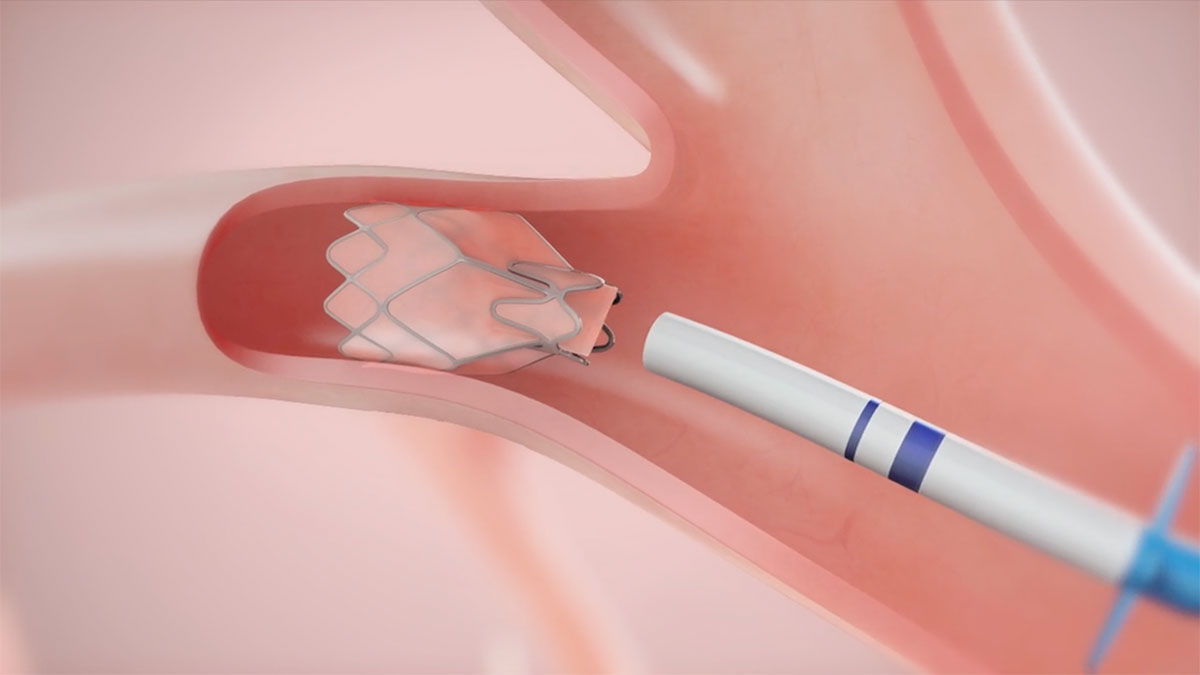 Pulmonx Ats 2020 Information For Treating Physicians
Pulmonx Ats 2020 Information For Treating Physicians
 Spiration Valve System P180007 Fda
Spiration Valve System P180007 Fda
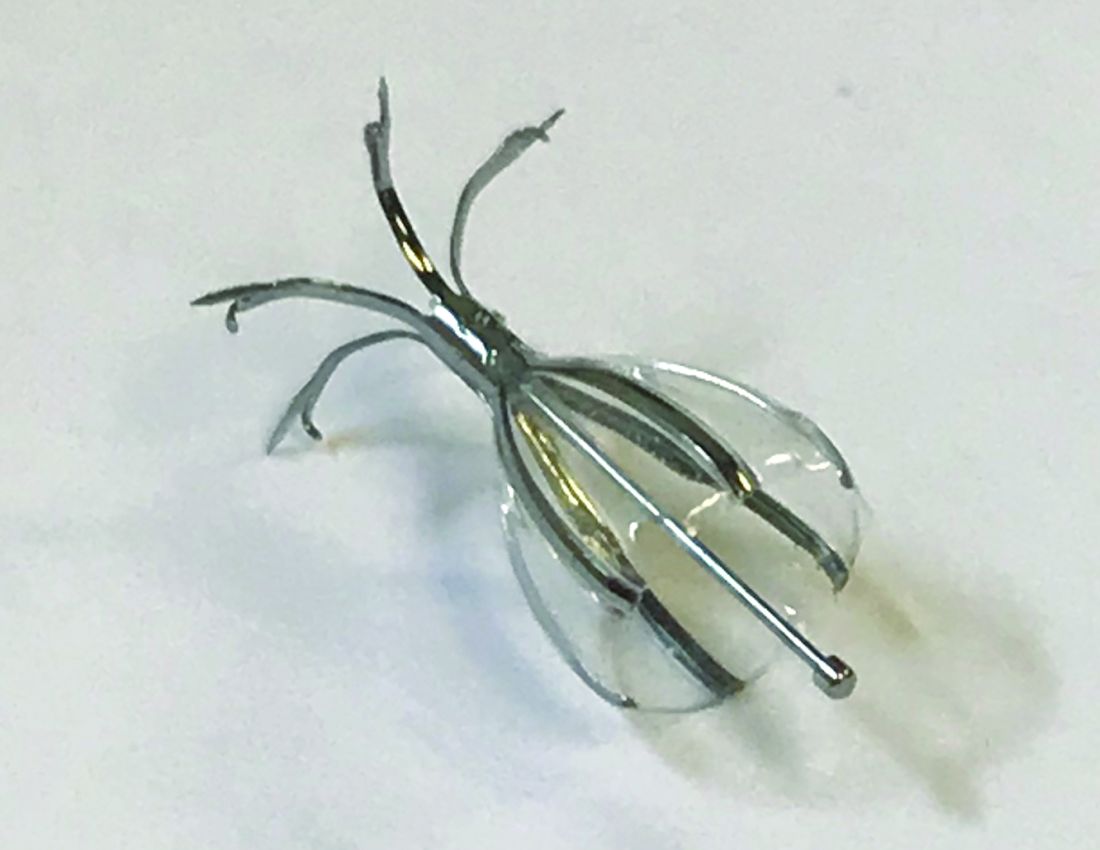 Endobronchial Valves For Lung Volume Reduction What Can We Offer Patients With Advanced Emphysema Chest Physician
Endobronchial Valves For Lung Volume Reduction What Can We Offer Patients With Advanced Emphysema Chest Physician
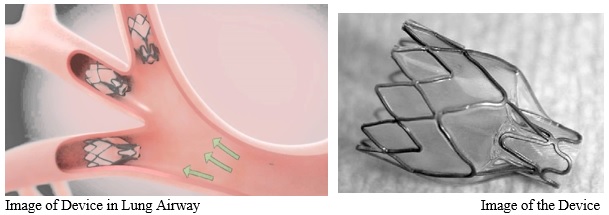
 Spiration Valve For Severe Emphysema Approved In U S Medgadget
Spiration Valve For Severe Emphysema Approved In U S Medgadget
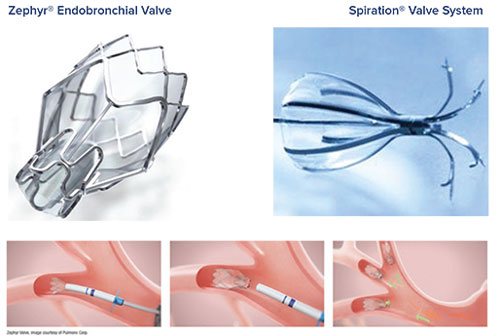 Groundbreaking Emphysema Treatment Inova
Groundbreaking Emphysema Treatment Inova
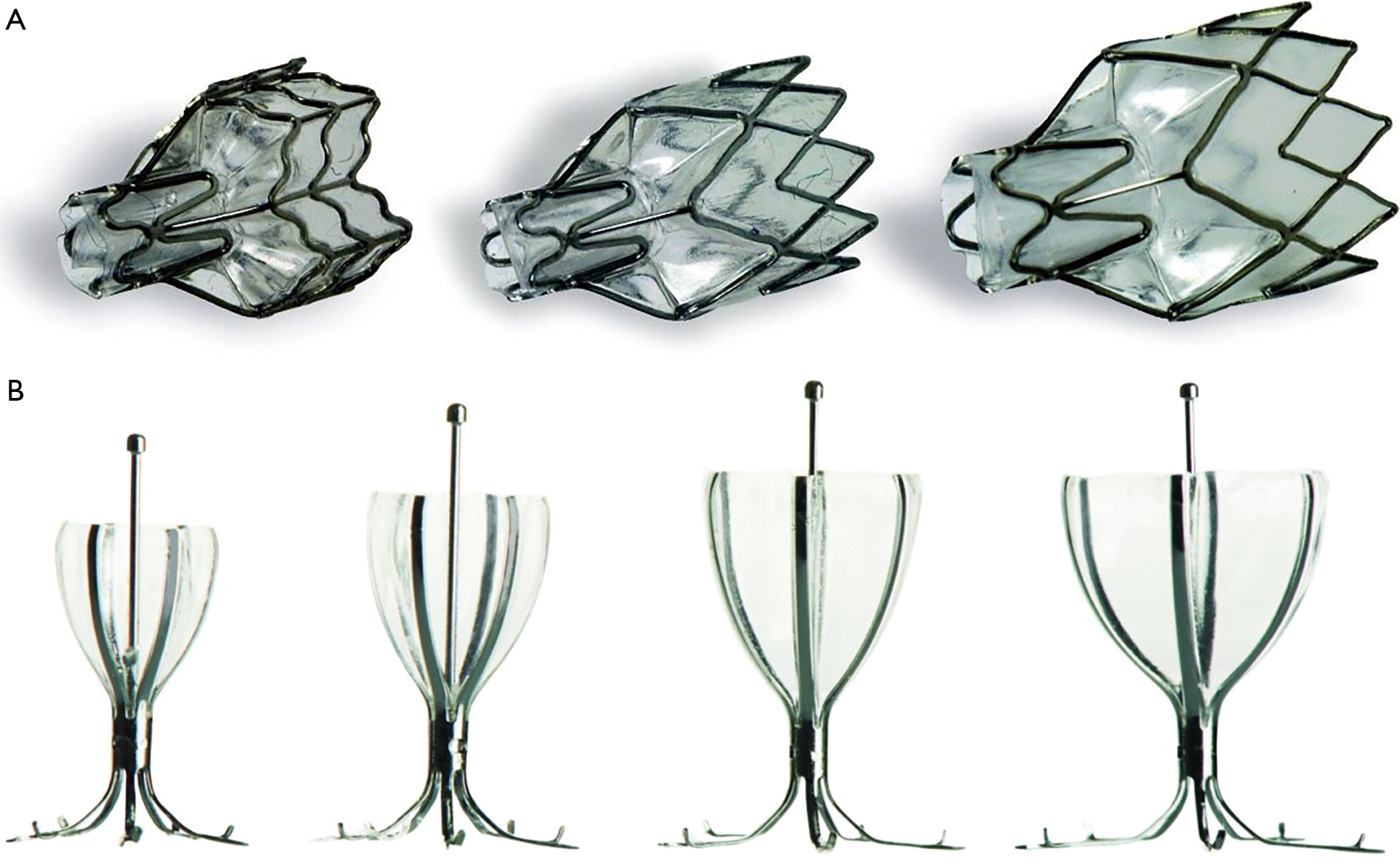 Valve Therapy In Patients With Emphysematous Type Of Chronic Obstructive Pulmonary Disease Copd From Randomized Trials To Patient Selection In Clinical Practice Valipour Journal Of Thoracic Disease
Valve Therapy In Patients With Emphysematous Type Of Chronic Obstructive Pulmonary Disease Copd From Randomized Trials To Patient Selection In Clinical Practice Valipour Journal Of Thoracic Disease
 Endobronchial Valves For Severe Emphysema European Respiratory Society
Endobronchial Valves For Severe Emphysema European Respiratory Society
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.