The PROPEL sinus implants are intended to maintain patency and locally deliver steroid to the sinus mucosa in patients 18 years of age following sinus surgery. Intersect ENT Inc.
 Propel Sinus Implants Princeton Nj Monroe Propel Sinus Implants
Propel Sinus Implants Princeton Nj Monroe Propel Sinus Implants
They are the first and only dissolvable steroid-releasing sinus implants approved by FDA according to the company.

Propel nasal stent. It is the first and only product on the market that targets the inflamed sinus tissues directly opening them up for improved drainage and a reduction in symptoms. Once placed the springlike construction expands to help prop open the sinuses after surgery. What is a PROPEL sinus stent.
The Propel sinus stent is small lightweight device that when implanted usually cannot even be felt. Contraindications include patients with confirmed hypersensitivity or intolerance to mometasone. Catalino et al 2011.
The stents then dissolve. Here are some additional benefits of PROPEL sinus stents. The devices work by propping open the sinuses and releasing the steroid directly into the sinus lining after surgery.
They are used by ENT otolaryngology surgeons to prop open the sinuses after sinus surgery and to locally deliver the corticosteroid mometasone furoate MF. The Propel Propel miniPropel Contour sinus implant is a bioabsorbable implant designed to maintain patency of the sinus cavity PropelPropel mini or sinus ostium Propel Contour. It delivers the drug in a controlled manner directly into the healing sinus tissues that need it.
What is a PROPEL sinus stent. In this video Dr. This helps to decrease inflammation swelling and prevent scarring.
The Propel Propel miniPropel Contour implant is manufactured from a synthetic bioabsorbable copolymer poly L-lactide-co-glycolide PLG. PROPEL sinus stents are small dissolvable and bio-compatible devices that are coated with an anti-inflammatory drug mometasone furoate. The Propel is clinically.
A smaller version of the drug delivery system Propel Mini is also available Intersect ENT 2019. The implant contains mometasone furoate active ingredient a synthetic. The PROPEL sinus implant is intended for use in patients 18 years of age following ethmoid sinus surgery to maintain patency thereby reducing the need for post-operative intervention such as surgical adhesion lysis andor use of oral steroids.
Intersect ENTs line of steroid-releasing stents include the Propel Propel Mini and Propel Contour. PROPEL sinus stent contains an anti-inflammatory medication. Persistent inflammation can risk return of sinus opening blockage PROPEL sinus stents can be inserted during sinus surgery to keep the sinuses passages open The stents are absorbable over time and eventually dissolve as the sinus openings.
The Propel Propel miniPropel Contour sinus implant is a bioabsorbable implant designed to maintain patency of the sinus cavity PropelPropel mini or sinus ostium Propel Contour. Propel - Sinus Stent - ENT Specialists. The Propel steroid-releasing implant is an alternative treatment for patients experiencing chronic sinus infections.
Oakley Smith demonstrates you the nasal stent is removed from the nose on the 7th day after the Rhinoplasty procedure. Absorption of medication when an implant is used. As the stent begins to gradually dissolve it releases steroid medication directly to the sinus lining.
XENT a global ear nose and throat ENT medical technology leader dedicated to transforming patient care today announced that the Centers for. The Propel Propel miniPropel Contour implant is manufactured from a synthetic bioabsorbable copolymer poly L-lactide-co-glycolide PLG. The Propel Intersect ENT Palo Alto CA a mometasone-eluting biodegradable implant is an example of a drug-eluting stent.
PROPEL for the ethmoid sinus PROPEL Mini for the ethmoid sinusfrontal sinus opening and PROPEL Contour for the frontalmaxillary sinus ostia. Propel is the first and only device clinically shown to keep the sinuses open after surgery precisely deliver medication directly into the healing tissues and then dissolve so that no removal is required. The stent device helps to keep sinuses open help deliver anti-inflammatory medication directly to the healing tissues and then dissolve.
They are used by ENT otolaryngology surgeons to prop open the sinuses after sinus surgery and to locally deliver the corticosteroid mometasone furoate MF. PROPEL sinus stents are small dissolvable and bio-compatible devices that are coated with an anti-inflammatory drug mometasone furoate. Propel Sinus Stent implant is clinically proven to improve surgical outcomes for chronic sinusitis sufferers.
The PROPEL sinus implant separates mucosal tissues provides stabilization of the middle turbinate prevents obstruction by adhesions and reduces edema. PROPEL is a sinus implant indicated to deliver a steroid drug locally to the ethmoid sinus and maintain the sinus opening in patients 18 years of age or olde.
 New Innovations In Sinus Surgery Technology My Sinusitis
New Innovations In Sinus Surgery Technology My Sinusitis
 Propel Sinus Stent Ascentist Ent
Propel Sinus Stent Ascentist Ent
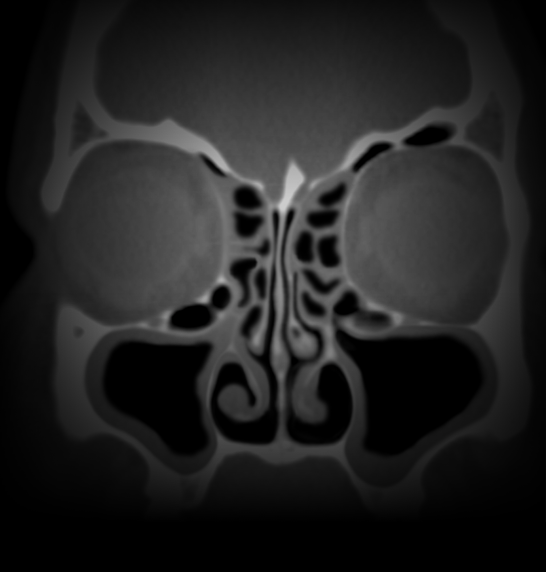 Propel Family Of Implants For Patients After Sinus Surgery Treat Chronic Rhinosinusitis With Propel Bioabsorbable Implants
Propel Family Of Implants For Patients After Sinus Surgery Treat Chronic Rhinosinusitis With Propel Bioabsorbable Implants
 Chronic Sinusitis Surgery Propel Sinus Stent My Sinusitis
Chronic Sinusitis Surgery Propel Sinus Stent My Sinusitis
 Propel Sinus Stent Ear Nose And Throat Doctors In Pembroke Pines Fl
Propel Sinus Stent Ear Nose And Throat Doctors In Pembroke Pines Fl
Propel Sinus Stent Ent Specialists
Propel Sinus Stent Glendale Beverly Hills Ca Chronic Sinusitis Treatment
 Propel Sinus Stent Animation Youtube
Propel Sinus Stent Animation Youtube
Propel Sinus Stent For Chronic Sinusitis
Propel Sinus Implant Treatment For Chronic Sinus Disease Joliet Morris New Lenox Ent Doctors
 Dissolvable Frontal Sinus Stent Implant Intersect Ent
Dissolvable Frontal Sinus Stent Implant Intersect Ent
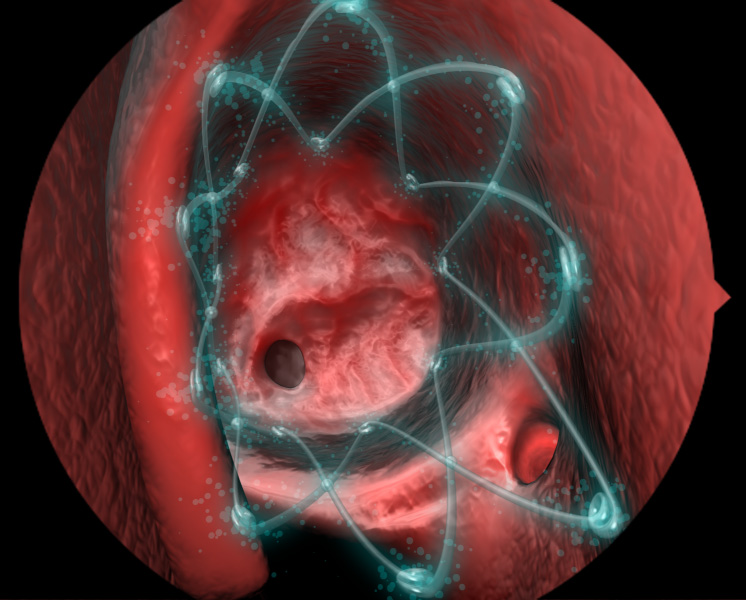 What Is Propel Sinus Implant Isaac Namdar Md
What Is Propel Sinus Implant Isaac Namdar Md
 Medical Monday Propel A Dissolvable Stent For Chronic Sinusitis Youtube
Medical Monday Propel A Dissolvable Stent For Chronic Sinusitis Youtube

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.