The physician must specifically order Retacrit. 8 Individualize anemia management with the multiple dosing and administration options of Aranesp Aranesp is also available in 200 300 and 500 mcg dose strengths.
 Safety Data Retacrit Epoetin Alfa Epbx For Hcps Safety Info
Safety Data Retacrit Epoetin Alfa Epbx For Hcps Safety Info
If youre interested in substituting your prescription for Epogen or Procrit with Retacrit youll need to ask your doctor to write you a new prescription.

Retacrit vs epogen. Epoetin alfa Epogen Procrit should be used with caution in patients with known albumin hypersensitivity. The rate of hemoglobin increase varies among patients and is dependent upon the dose of Epogen Procrit or Retacrit being administered. RETACRIT showed no clinically meaningful difference in mean weekly dose compared to Epogen 2 The mean weekly dose needed to maintain Hb target level in CKD patients during the last 4 weeks of treatment had no clinically meaningful difference between treatment groups in either study.
May 15 2018 The US. Retacrit cannot be interchanged with Epogen at the pharmacy level. The primary endpoint was the proportion of time patients hemoglobin was 9-11 gdL during weeks 17-24.
Due to effective plasma donor screening for prior exposure to certain viruses testing for the presence of viruses and manufacturing processes designed to reduce the risk of transmitting viral infection the risk of transmission of infectious agents associated with epoetin alfa products formulated. Retacrit is a biosimilar to Epogen. RETACRIT the First US.
The 10 150 300 and 500 mcg dose strengths are only available. The recommended RETACRIT regimens are. Epogen Procrit and Retacrit increase the reticulocyte count within 10 days of initiation followed by increases in the RBC count hemoglobin and hematocrit usually within 2 to 6 weeks.
600 Unitskg subcutaneously in 4 doses administered 21 14 and 7. FDA Approved Indications Epogen Procrit and Retacrit are indicatedfor. Design setting participants measurements.
1 to switch to IV RetacritTM or continue standard-of-care Epogen for 24 weeks using analogous versions of the FMCNA ESA-dosing algorithm. RETACRIT is now the first and only. Retacrit is the first FDA-approved biosimilar to Epogen.
The FDA further noted that although Retacrit has been approved as a biosimilar it has not been approved as interchangeable with Epogen. Retacrit epoetin alfa-epbx from Hospira and Epogen epoetin alfa from Amgen are both erythropoiesis-stimulating agents ESA used to help improve red blood cell production. O Chronic kidney disease CKD in patients on dialysis and not on dialysis.
Half Life The half-life of a drug is the time taken for the plasma concentration of a drug to reduce to half its original value. This study was conducted to compare the safety and efficacy of intravenous epoetin alfa-epbx an epoetin alfa biosimilar to epoetin alfa in patients on hemodialysis with ESKD and anemia. On May 15 2018 the US.
Retacrit is a biosimilar which means its active ingredients arent interchangeable with those in Epogen or Procrit. NYSEPFE today announced the United States US Food and Drug Administration FDA approved RETACRIT epoetin alfa-epbx a biosimilar to Epogen and Procrit epoetin alfa1 for all indications of the reference product. 300 Unitskg per day subcutaneously for 15 days total.
Retacrit epoetin alfa-epbx New biosimilar approval On May 15 2018 the FDA announced the approval of Retacrit epoetin alfa-epbx HospiraPfizers biosimilar to Amgens Epogen epoetin alfa and Janssens Procrit epoetin alfa. CSA Schedule View glossary of terms. Administered daily for 10 days before surgery on the day of surgery and for 4 days after surgery.
Food and Drug Administration today approved Retacrit epoetin alfa-epbx as a biosimilar to EpogenProcrit epoetin alfa for the treatment of anemia. Patients with anemia and chronic kidney disease undergoing maintenance hemodialysis and receiving routine intravenous IV Epogen were randomized 1. Epoetin alfa Epogen Procrit and its biosimilar epoetin alfa-epbx Retacrit are erythropoiesis-stimulating agents ESAs.
In this 24-week multicenter double-blind comparative efficacy and safety study 612 patients on hemodialysis with ESKD and anemia. Retacrit is the first epoetin alfa biosimilar to be approved by the FDA and just the tenth biosimilar approved overall. On May 15 2018 the Food and Drug Administration approved Retacrit epoetin alfa-epbx Hospira Inc a subsidiary of Pfizer Inc as a biosimilar to EpogenProcrit epoetin alfa Amgen Inc for.
Biosimilar Erythropoiesis-Stimulating Agent ESA Now Approved Across All Indications Pfizer Inc. Other epoetin alfa brands include. Treatment of anemia due to.
Food and Drug Administration FDA approved Retacritepoetin alfa-epbx Hospira Inc a subsidiary of Pfizer Inc as a biosimilar to EpogenProcrit epoetin alfa Amgen Inc for the treatment of anemia due to chronic kidney disease in patients on dialysis and not on dialysis use of zidovudine in patients with HIV infection and the effects of.
 Intravenous Epoetin Alfa Epbx Versus Epoetin Alfa For Treatment Of Anemia In End Stage Kidney Disease American Society Of Nephrology
Intravenous Epoetin Alfa Epbx Versus Epoetin Alfa For Treatment Of Anemia In End Stage Kidney Disease American Society Of Nephrology
 Long Term Safety Of Epoetin Alfa Epbx For The Treatment Of Anemia In Eskd Pooled Analyses Of Randomized And Open Label Studies Kidney Medicine
Long Term Safety Of Epoetin Alfa Epbx For The Treatment Of Anemia In Eskd Pooled Analyses Of Randomized And Open Label Studies Kidney Medicine
 Clinical Data Retacrit Epoetin Alfa Epbx For Hcps Safety Info
Clinical Data Retacrit Epoetin Alfa Epbx For Hcps Safety Info
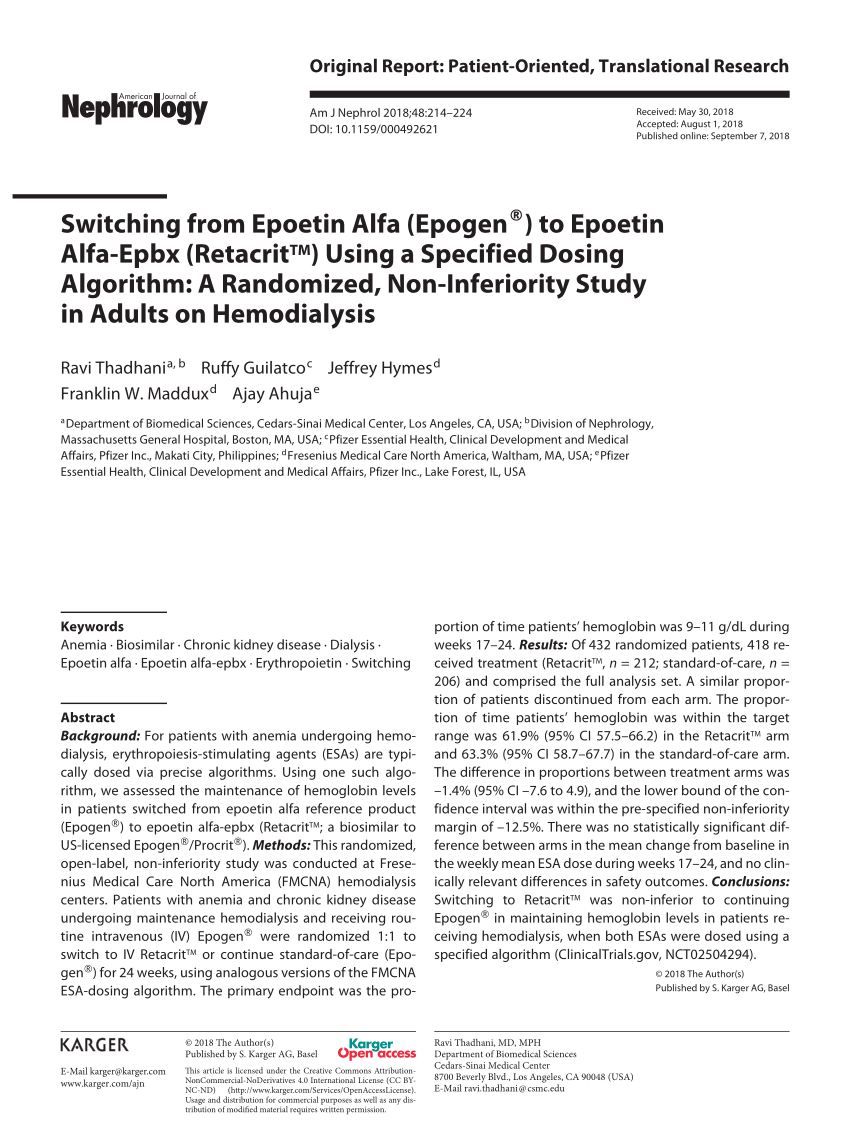 Pdf Switching From Epoetin Alfa Epogen To Epoetin Alfa Epbx Retacrittm Using A Specified Dosing Algorithm A Randomized Non Inferiority Study In Adults On Hemodialysis
Pdf Switching From Epoetin Alfa Epogen To Epoetin Alfa Epbx Retacrittm Using A Specified Dosing Algorithm A Randomized Non Inferiority Study In Adults On Hemodialysis
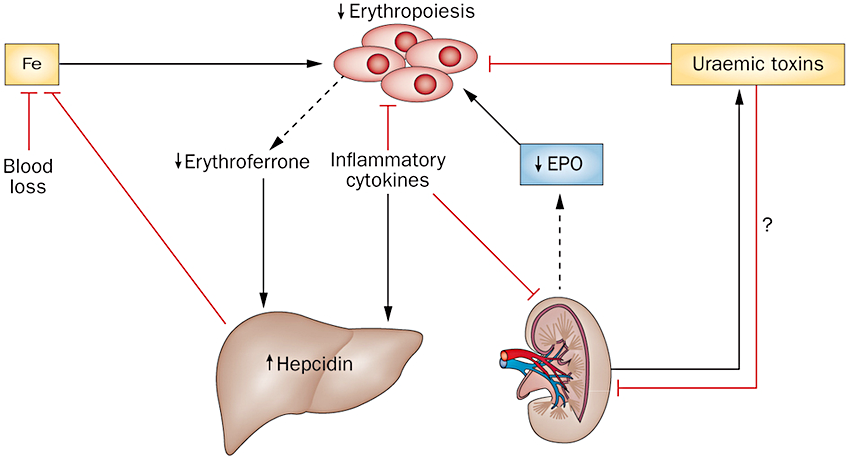 New Fda Approval Retacrit To Improve Anemia Of Kidney Disease Gt Health Endocrinology
New Fda Approval Retacrit To Improve Anemia Of Kidney Disease Gt Health Endocrinology
Https Www Ema Europa Eu Documents Scientific Discussion Retacrit Epar Scientific Discussion En Pdf
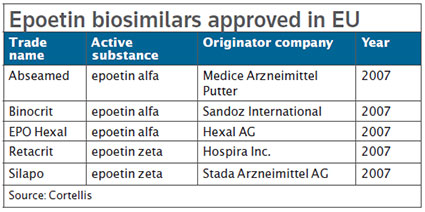 Hospira S Retacrit Next Biosimilar On Tap For Fda Bioworld
Hospira S Retacrit Next Biosimilar On Tap For Fda Bioworld
 Safety Data Retacrit Epoetin Alfa Epbx For Hcps Safety Info
Safety Data Retacrit Epoetin Alfa Epbx For Hcps Safety Info
 Fda Approves First Epoetin Alfa Biosimilar Qtc Recruitment
Fda Approves First Epoetin Alfa Biosimilar Qtc Recruitment
 Intravenous Epoetin Alfa Epbx Versus Epoetin Alfa For Treatment Of Anemia In End Stage Kidney Disease American Society Of Nephrology
Intravenous Epoetin Alfa Epbx Versus Epoetin Alfa For Treatment Of Anemia In End Stage Kidney Disease American Society Of Nephrology
 Clinical Data Retacrit Epoetin Alfa Epbx For Hcps Safety Info
Clinical Data Retacrit Epoetin Alfa Epbx For Hcps Safety Info
 References In Current And Upcoming Erythropoiesis Stimulating Agents Iron Products And Other Novel Anemia Medications Advances In Chronic Kidney Disease
References In Current And Upcoming Erythropoiesis Stimulating Agents Iron Products And Other Novel Anemia Medications Advances In Chronic Kidney Disease
 Clinical Data Retacrit Epoetin Alfa Epbx For Hcps Safety Info
Clinical Data Retacrit Epoetin Alfa Epbx For Hcps Safety Info
 Biosimilar Retacrit An Erythropoiesis Stimulating Agent Now Available Mpr
Biosimilar Retacrit An Erythropoiesis Stimulating Agent Now Available Mpr
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.