The recommended dosage is one drop in. The recommended dosage is one drop in.
 Rhopressa Fda Prescribing Information Side Effects And Uses
Rhopressa Fda Prescribing Information Side Effects And Uses
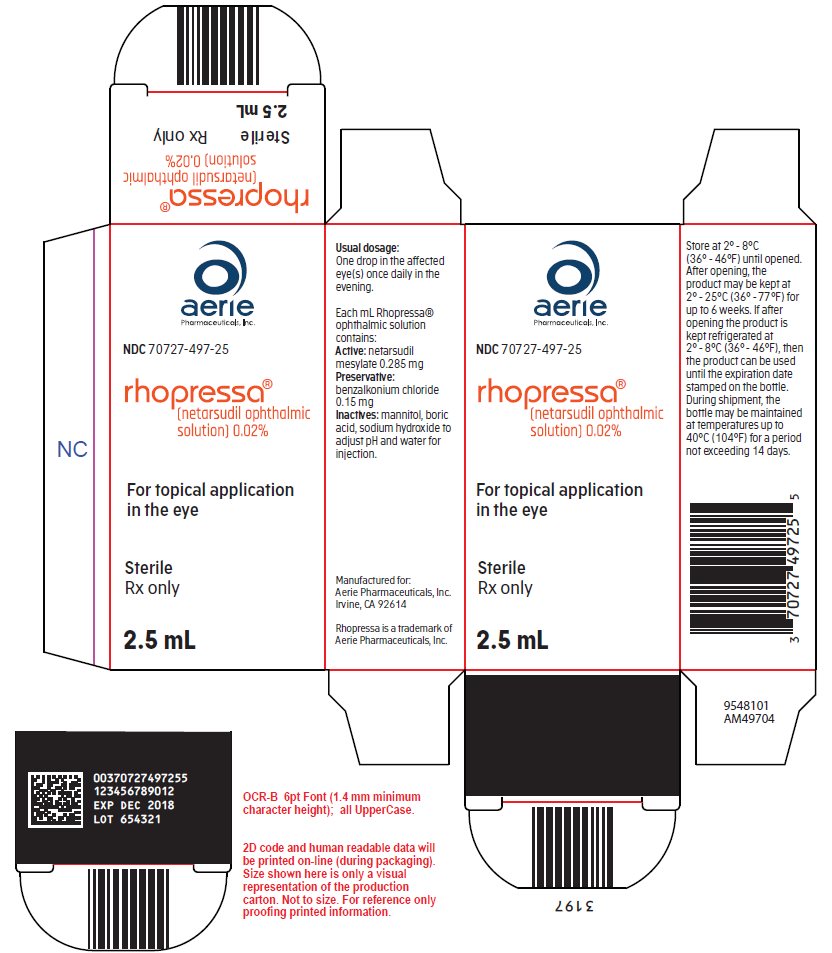
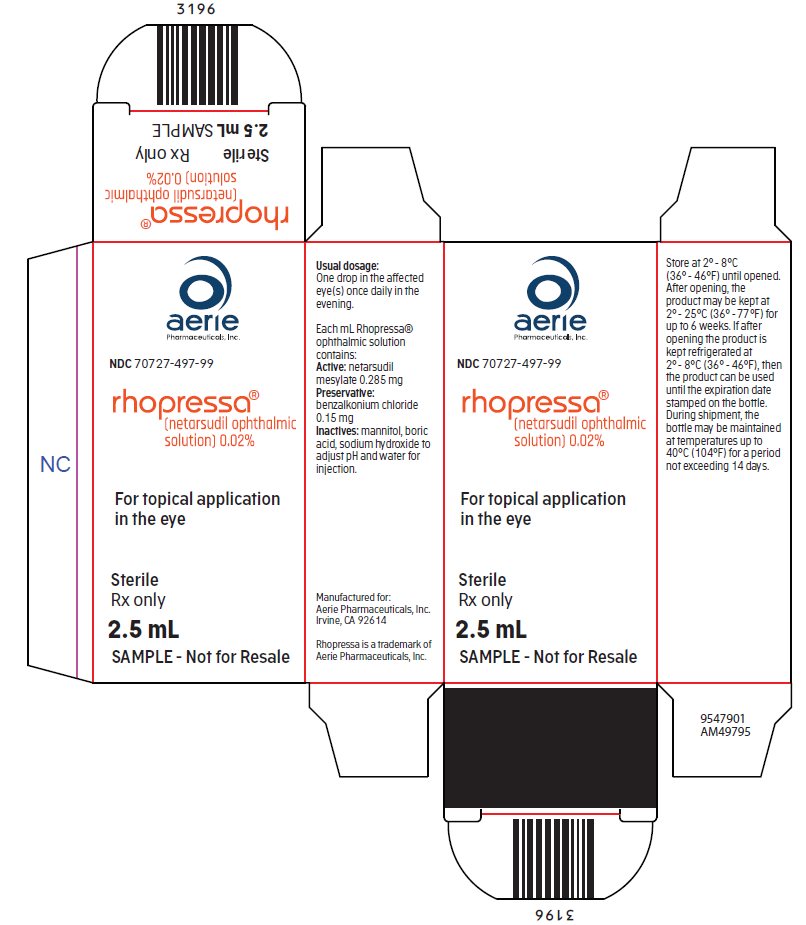
Each mL of Rhopressa contains 02 mg of netarsudil equivalent to 028 mg of netarsudil dimesylate.

Rhopressa package insert. 2 DOSAGE AND ADMINISTRATION 21 General Dosing Information For ophthalmic intravitreal injection. 3 53 Intraocular Inflammation VYZULTA should be used with caution in patients with a history of intraocular inflammation iritisuveitis and should generally not be used in patients with active intraocular inflammation as it may exacerbate this condition. RHOPRESSA netarsudil ophthalmic solution 002 02 mg per mL is supplied sterile in opaque white low density polyethylene bottles and tips with white polypropylene caps.
Initial Criteria approved for 12 months subject to formulary changes. 2 DOSAGE AND ADMINISTRATION. One bottle 25 mL or 5 mL per 30 days RHOPRESSA.
RHOPRESSA netarsudil ophthalmic solution 002 is indicated for the reduction of elevated intraocular pressure IOP in patients with open-angle glaucoma or ocular hypertension. Rhopressa netarsudil demethylase is a Rho kinase inhibitor indicated for the reduction of elevated intraocular pressure IOP in patients with open-angle glaucoma or ocular hypertension. Rhopressa netarsudil demethylase is believed to reduce IOP by increasing the outflow of aqueous humor through the trabecular meshwork route.
The inactive ingredients are. Indication for Prior Authorization. The recommended dosage is one drop in the affected eye s once daily in the evening.
Rhopressa netarsudil ophthalmic solution 002 is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. How is this drug used. Benzalkonium chloride 0015 is added as a preservative.
If one dose is missed treatment should. The exact mechanism is unknown. Patient pay amount may vary dependent upon commercial insurance coverage for ROCKLATAN or RHOPRESSA.
Netarsudil 002 Rhopressa Pharmacology. One drop of RHOPRESSA is applied in the affected eye once daily. FULL PRESCRIBING INFORMATION 1 INDICATIONSAND USAGE BEOVU is indicated for the treatment of Neovascular Wet Age-related Macular Degeneration AMD.
Eligible commercially insured patients may pay as little as 25 per 30-day 60-day or 90-day supply. Rhopressa netarsudil ophthalmic solution 002 is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Indicated for the reduction of elevated intraocular pressure IOP in patients with open-angle glaucoma or ocular hypertension.
Netarsudil is a rho kinase inhibitor which is believed to reduce IOP by increasing the outflow of aqueous humor through the trabecular meshwork. Patient is 18 years of age or older AND. 2 DOSAGE AND ADMINISTRATION.
TIMOPTIC Package Insert 4 In a study of plasma drug concentration in six subjects the systemic exposure to timolol was determined following twice daily administration of TIMOPTIC 05. Listed in the package insert or generally accepted by peers and the reason for additional services is not justified by submitted documentation. Diagnosis of open-angle glaucoma or ocular hypertension AND.
Patients with questions about the ROCKLATAN or RHOPRESSA Savings offer should call 1-844-807-9706. Netarsudil solution Rhopressa SELF ADMINISTRATION- OPHTHALMIC. The mean peak plasma concentration following morning dosing was 046 ngmL and following afternoon dosing was 035 ngmL.
Boric acid mannitol sodium hydroxide to adjust pH and water for injection. Rocklatan netarsudil and latanoprost ophthalmic solution 0020005 is a prescription medication for the treatment of high eye pressureintraocular pressure IOP in people with open-angle glaucoma or ocular hypertension. RHOPRESSA is a drug for reducing elevated intraocular pressure when the pressure inside the eye is too high.
25 mL fill in a 4 mL container NDC 70727-497-25. One bottle 25 mL per 30 days. RHOPRESSA netarsudil ophthalmic solution 002 is indicated for the reduction of elevated intraocular pressure IOP in patients with open-angle glaucoma or ocular hypertension.
The recommended dosage is one drop in the affected eyes once daily in the evening.

 These Highlights Do Not Include All The Information Needed To Use Rhopressa Safely And Effectively See Full Prescribing Information For Rhopressa Rhopressa Netarsudil Ophthalmic Solution 0 02 For Topical Ophthalmic Use Initial U S
These Highlights Do Not Include All The Information Needed To Use Rhopressa Safely And Effectively See Full Prescribing Information For Rhopressa Rhopressa Netarsudil Ophthalmic Solution 0 02 For Topical Ophthalmic Use Initial U S
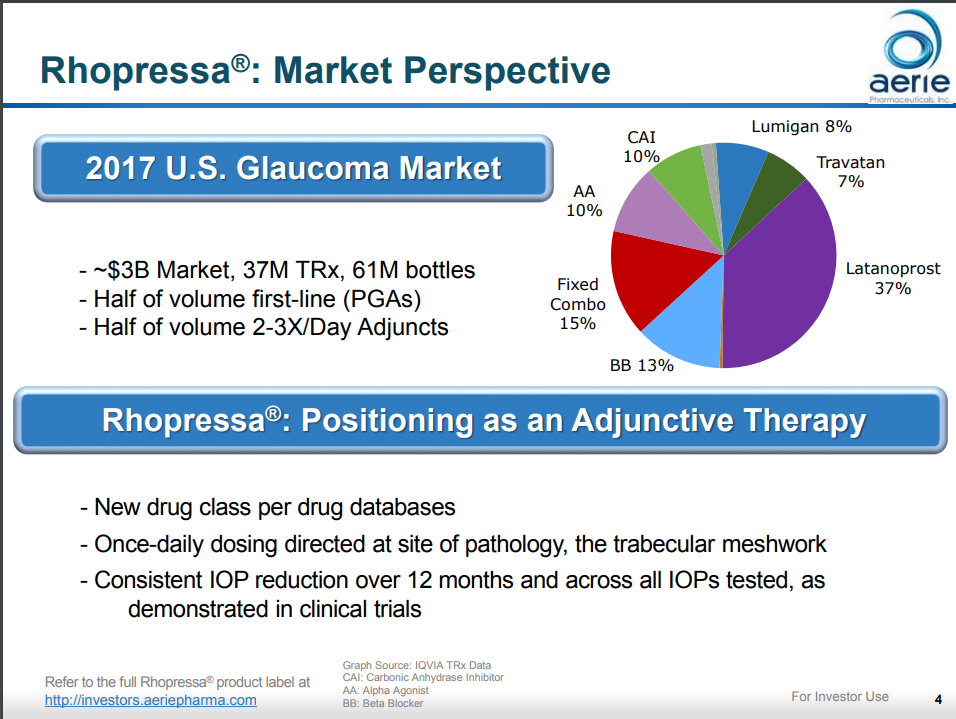
 Crstoday Newly Approved Glaucoma Therapy Increases Options For Patients And Providers
Crstoday Newly Approved Glaucoma Therapy Increases Options For Patients And Providers
 Dosing Safety Rhopressa Netarsudil Ophthalmic Solution 0 02
Dosing Safety Rhopressa Netarsudil Ophthalmic Solution 0 02
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2017 208254lbl Pdf
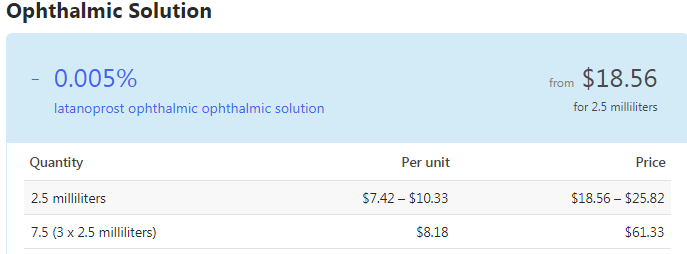
Rhopressa Medicare Coverage And Co Pay Details Goodrx
 Rhopressa Netarsudil Topical Ophthalmic Use Uses Dosage Side Effects Interactions Warning
Rhopressa Netarsudil Topical Ophthalmic Use Uses Dosage Side Effects Interactions Warning
 Aerie Pharmaceuticals Positive Rhopressa Launch May Mark The Stock Price Peak Nasdaq Aeri Seeking Alpha
Aerie Pharmaceuticals Positive Rhopressa Launch May Mark The Stock Price Peak Nasdaq Aeri Seeking Alpha
 Rhopressa Fda Prescribing Information Side Effects And Uses
Rhopressa Fda Prescribing Information Side Effects And Uses
 Savings And Support For Rhopressa Rhopressa Netarsudil Ophthalmic Solution 0 02
Savings And Support For Rhopressa Rhopressa Netarsudil Ophthalmic Solution 0 02
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2017 208254lbl Pdf
 These Highlights Do Not Include All The Information Needed To Use Rhopressa Safely And Effectively See Full Prescribing Information For Rhopressa Rhopressa Netarsudil Ophthalmic Solution 0 02 For Topical Ophthalmic Use Initial U S
These Highlights Do Not Include All The Information Needed To Use Rhopressa Safely And Effectively See Full Prescribing Information For Rhopressa Rhopressa Netarsudil Ophthalmic Solution 0 02 For Topical Ophthalmic Use Initial U S
 Aerie Pharmaceuticals Positive Rhopressa Launch May Mark The Stock Price Peak Nasdaq Aeri Seeking Alpha
Aerie Pharmaceuticals Positive Rhopressa Launch May Mark The Stock Price Peak Nasdaq Aeri Seeking Alpha

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.