The JAK2 V617F exon 14 mutation analysis can be used in conjunction with bone marrow histology and cytogenetic analysis to assist in the diagnosis of myeloproliferative neoplasma MPN. JAK2 exon 12 mutations as well as other mutations in exons 13 and 14 have been reported in rare cases of non-CML MPDs negative for V617F.
 Results Of Analyses For Jak2v617f Jak2 Exon 12 And Mplw515 Mutations Download Table
Results Of Analyses For Jak2v617f Jak2 Exon 12 And Mplw515 Mutations Download Table
17851549 PubMed - indexed for MEDLINE Publication Types.
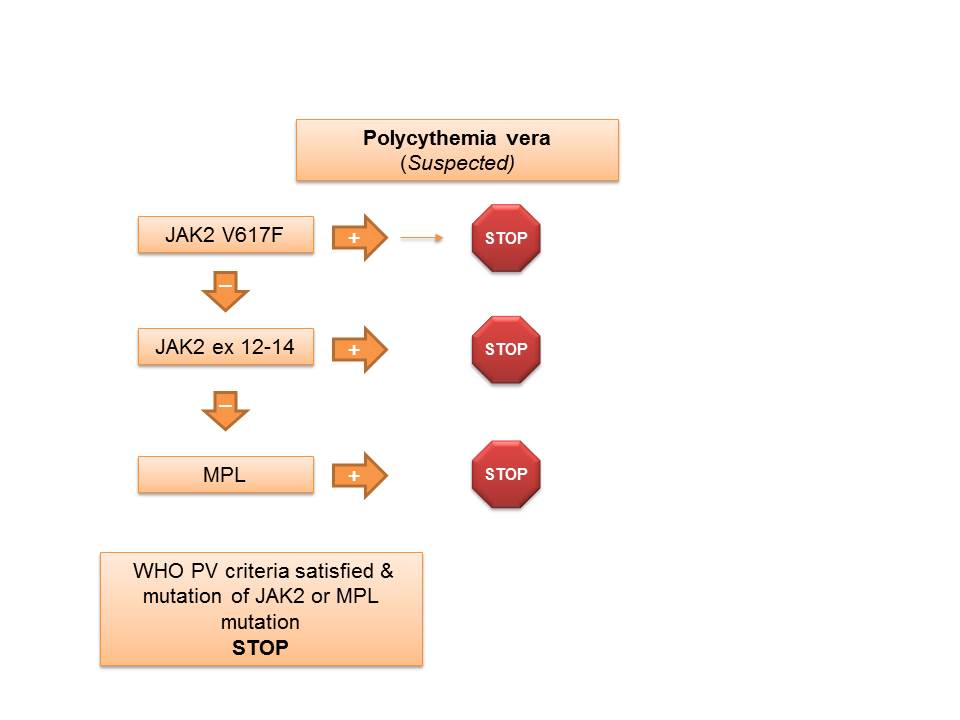
Jak2 exon 12 14 mutation analysis. Detects mutations in exons 12 and 13 of the JAK2 gene in myeloproliferative neoplasms MPNs. These two groups patients with JAK2 exon 12 mutations presented at a significantly younger age than patients with JAK2V617F mutations. The exon 14 analysis included 163 patients with polycythemia vera secondary erythrocytoses essential thrombocythemia or secondary thrombocytoses and 126 healthy subjects.
Testing is performed on plasma for increased sensitivity whenever possible. Analysis of the exon 12 and 14 mutations of the JAK2 gene in Philadelphia chromosome-positive leukemia. The JAK2 mutation test is typically ordered as a follow-up test.
RT-PCR and bi-directional sequencing to detect non-V617F mutations in exons 12-14 and most of exon 15 corresponding to the majority of the JAK2 pseudokinase domain. Compared with the V617F mutation exon 12 mutations result in stronger ligand-independent signaling through JAK2. Exon deletion mutations are detectable.
Exon 12-14 Mutation Analysis may be ordered separately with concurrent V617F testing by reflex after negative V617F. These mutations are associated with myeloproliferative. This test is designed to detect gene mutations only.
V617F analysis is recommended before or concurrently with this test. Several of the exon 12 mutations have been shown to have biologic effects similar to those caused by the V617F mutation such that it is currently assumed other nonpolymorphic mutations. Analysis of the exon 12 and 14 mutations of the JAK2 gene in Philadelphia chromosome-positive leukemia.
Later on JAK2 exon 12 and 14 was amplified by conventional PCR in V617F negative patients and subjected to sequence analysis. It is not intended to detect copy number changes or gene rearrangements. The exon 14 analysis included 163 patients with polycythemia vera secondary erythrocytoses essential thrombocythemia or secondary.
2 We were unable to obtain clinical data on our referral samples as they were received from diverse hospitals. This test will assess for the JAK2 V617F exon 14 mutation first and will reflex to JAK2 exon 12 to 15 mutation analysis when the JAK2 V617F mutation is negative. Analysis of the subsequent data reported in the literature for 134 exon 12-mutated patients confirms this initial observation.
A total of 03 mutated sites in exon 12 were detected in only two V617F-negative patients 25 40. The JAK2 V617F mutation is found in almost all patients with polycythemia vera PV and in nearly one-half of those with idiopathic myelofibrosis IMF and with essential thrombocythemia ET. The JAK2 V617F exon 14 mutation analysis can be used in conjunction with bone marrow histology and cytogenetic analysis to assist in the diagnosis of myeloproliferative neoplasms MPN.
Cutoff value based on assay sensitivity. JAK2 Mutation Analysis Test. JAK2 mutations are important criteria for the diagnosis of Philadelphia chromosome-negative myeloproliferative neoplasms.
These mutations include point mutations and small insertions or deletions. F537FI found in both patients and R528RT which is a novel mutation. 7 - 10 days Reference Ranges.
This is characterized by erythrocytosis with a raised hematocrit and hemoglobin level reduced serum EPO levels and EPOhypersensitive erythroid progenitors but often lacks the proliferation of cells of the granulocytic or megakaryocytic lineages generally observed in patients with. Exon 12-13 Mutation Analysis may be ordered separately with concurrent V617F testing by reflex after negative V617F. All three substitutions were heterozygous ie.
Testing is performed on plasma for increased sensitivity whenever possible. JAK2 Exon 12 Mutation Analysis - This DNA-based assay tests leukocytes from blood or bone marrow aspirate for mutations in exon 12 of JAK2 using an advanced DNA sequencing method. Again there is no differ-ence in incidence according to gender 70 female 64 male.
Inami M Yamaguchi H Hasegawa S Mitamura Y Kosaka F Kobayashi A Kimura S Dan K Inokuchi K. RT-PCR and bi-directional sequencing to detect non-V617F mutations in exons 12-14 and most of exon 15 corresponding to the majority of the JAK2 pseudokinase domain. Over 50 different mutations have now been reported within exons 12 through 15 of JAK2 and essentially all of the non-V617F mutations have been identified in polycythemia vera.
We aimed to assess JAK2 exon 14 and exon 12 mutations by high-resolution melting HRM analysis which allows variation screening. JAK2 exon 12 mutation screening is indicated in the presence of JAK2 V617F-negative erythrocytosis that is associated with a low serum erythropoietin level or in the presence of characteristic symptoms of PV even if the complete blood count is not suggestive of the condition. We aimed to assess JAK2 exon 14 and exon 12 mutations by high-resolution melting HRM analysis which allows variation screening.
Exon 12 mutations generate higher levels of JAK2 and ERK1 and ERK2 phosphorylation. V617F analysis is recommended before or concurrently with this test. Patients with a JAK2 exon 12 mutation present with a hematologic disorder consistent with the diagnosis of PV.
A JAK2 Mutational Analysis is usually recommended when a physician suspects a myeloproliferative disorder. M Inami 1 H Yamaguchi 1 S Hasegawa 2 Y. In order to perform the JAK2 mutation test blood sample is required which is drawn using a syringe inserted into a superficial vein of the arm.
Exon deletion mutations are detectable. Most testing for JAK2 mutations is performed by analyzing the genomic DNA of the JAK2 gene.