Eli Lilly and Company. 2 Gastric Cancer The recommended.
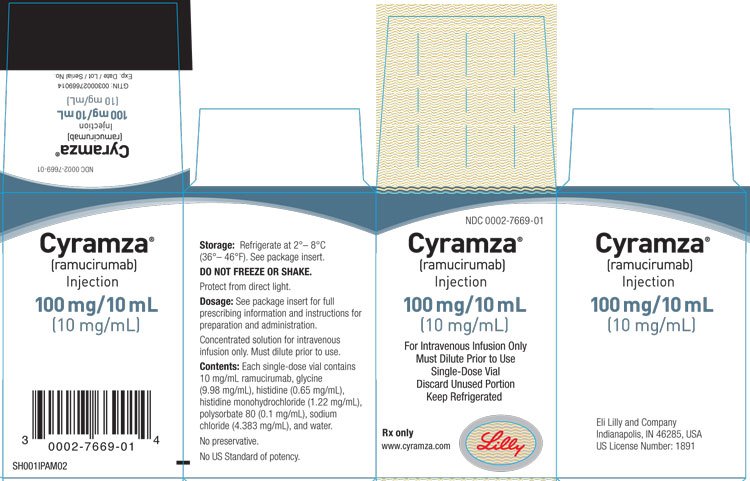 Cyramza Fda Prescribing Information Side Effects And Uses
Cyramza Fda Prescribing Information Side Effects And Uses
Cyramza monotherapy is indicated for the treatment of adult patients with advanced gastric cancer or gastro-oesophageal junction adenocarcinoma with disease progression after prior platinum or fluoropyrimidine chemotherapy for whom treatment in combination with paclitaxel is not appropriate see section 51.

Cyramza fda label. This FDA approval for CYRAMZA is based on the Phase III RAINBOW trial which compared CYRAMZA plus paclitaxel to placebo plus paclitaxel. CYRAMZA is supplied at a concentration of 10 mgmL in either 100 mg 10 mL or 500 mg 50 mL single-dose vials. Cyramza in combination with docetaxel is indicated for the treatment of patients with metastatic NSCLC with disease progression on or after platinum-based chemotherapy.
Cyramza gained FDA approval for use in combination. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving Cyramza. Eli Lilly and Company LLY announced a label expansion of its oncology drug Cyramza in the US.
13 Colorectal Cancer. The labeling for CYRAMZA contains a Boxed Warning regarding increased risk of hemorrhage including severe and sometimes fatal hemorrhagic events. FDA-approved therapy for these aberrations prior to receiving CYRAMZA.
CYRAMZA ramucirumab injection for intravenous use is a sterile preservative-free clear to slightly opalescent and colorless to slightly yellow solution. For detailed information on the use of Cyramza in all conditions see the package leaflet or contact your doctor or pharmacist. Gastric Cancer Non-Small Cell Lung Cancer Colorectal Cancer Hepatocellular Carcinoma.
CYRAMZA increased the risk of hemorrhage including severe and sometimes fatal hemorrhagic events. CYRAMZA ramucirumab injection for intravenous infusion Initial US. CYRAMZA in combination with FOLFIRI irinotecan folinic acid and fluorouracil is.
12 For intravenous infusion only. Efficacy endpoints in the trial included the major efficacy outcome measure of overall survival and the supportive efficacy outcome measures of progression-free survival and objective response rate. For most cancers it is used in combination with other medicines.
The updated CYRAMZA labeling continues to provide important information on these specific risks as well as other adverse events to physicians and the patients that work in. The FDA has removed the boxed warning from the CYRAMZA labeling which highlighted warnings pertaining to hemorrhage gastrointestinal perforation and impaired wound healing. Permanently discontinue CYRAMZA in patients who experience severe.
FDA label information for this drug is available at DailyMed. It is used with FOLFIRI in patients whose disease has gotten worse during or after treatment with bevacizumab oxaliplatin and a fluoropyrimidine. Colorectal cancer that has metastasized.
Yes First approved April 21 2014 Brand name. Cyramza contains the active substance ramucirumab. CYRAMZA is formulated in glycine 998 mgmL histidine 065 mgmL histidine.
The FDA advisers based their recommendation on Lillys Relay trial in which it reported that adding Cyramza to Tarceva reduced the risk of disease progression or death by 40 over Tarceva alone. HEMORRHAGE See full prescribing information for complete boxed warning. Do not administer as an intravenous push or bolus.
Ramucirumab is approved to be used alone or with other drugs to treat. FDA approves ramucirumab for hepatocellular carcinoma On May 10 2019 the Food and Drug Administration approved ramucirumab CYRAMZA Eli Lilly and Company as a single agent for hepatocellular. Cyramza is for use in cancers that are usually advanced or have spread despite other treatment.
13 Colorectal Cancer CYRAMZA in combination with FOLFIRI irinotecan folinic acid and fluorouracil is. INDIANAPOLIS dpa-AFX - Eli Lilly and Company LLY announced the FDA has approved CYRAMZA for the treatment of patients with hepatocellular carcinoma who have a high alpha-fetoprotein and have. On May 29 2020 the Food and Drug Administration approved ramucirumab CYRAMZA Eli Lilly and Company in combination with erlotinib for first-line treatment of metastatic non-small cell lung.
Progression on FDA-approved therapy for these aberrations prior to receiving CYRAMZA. Progression on FDA-approved therapy for these aberrations prior to receiving CYRAMZA.