Xyosted autoinjector testosterone enanthate. Xyosted a product of Antares Pharma Inc is a single-use disposable auto-injector that dispenses testosterone enanthate.
 Prescribing Testosterone Xyosted Health Care Provider
Prescribing Testosterone Xyosted Health Care Provider
Standards of Care for the Heath of Transsexual Transgender and Gender Nonconforming People.
Xyosted package insert. Tes-TOS-ter-one Brand name s Xyosted. Xyosted testosterone enanthate injection package insert. Testopel Pellets package insert.
If this occurs the drug should be discontinued. Never give your XYOSTED to anyone else. XYOSTED is a controlled substance CIII because it contains testosterone that can be a target for people who abuse prescription medicines.
Accessed October 22 2020. Testosterone Topical Solution package insert. Hypercalcemia may occur in immobilized patients.
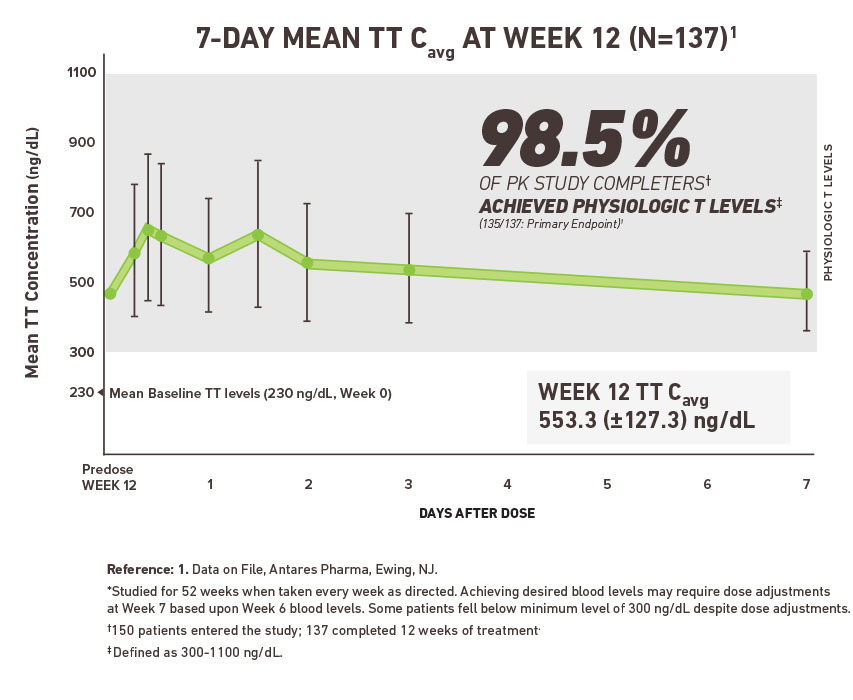
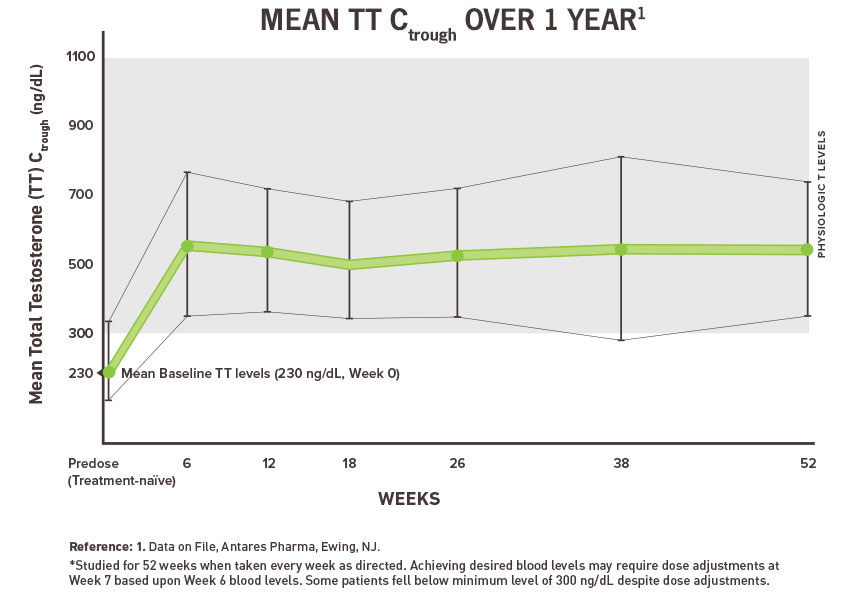
Xyosted testosterone enanthate will be approved based on all of the following. Prolonged use of high doses of androgens principally the 17-α alkyl-androgens has been associated with development of hepatic adenomas hepatocellular carcinoma and peliosis hepatis all potentially life-threatening complications. Xyosted testosterone enanthate injection October 8 2019 Please see the full Prescribing Information including Boxed Warning and Medication Guide here.
Improper use of Xyosted. Xyosted is the first FDA-approved subcutaneous testosterone enanthate product for testosterone replacement therapy in adult males. Testosterone - subcutaneous injection.
Keep your XYOSTED in a safe place to protect it. Pharmacy Studies of customers satisfaction with their pharmacy. Humana Pharmacys received the highest score among mail order pharmacies in the JD.
The World Professional Association for Transgender Health. The company must demonstrate that treatment with XYOSTED is not related to the side effects to a clinically relevant degree or to determine a dose. Your risk may be higher if you have a medical history of high blood pressure heart disease or stroke.
XYOSTED testosterone enanthate injection is an androgen indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous. Program Prior Authorization Change Control Date Change 92009. On October 1 2018 the US.
The covered person must be diagnosed with gender dysphoria as defined by the current version of the Diagnostic and Statistical Manual of Mental Disorders DSM. Find patient medical information for Xyosted subcutaneous on WebMD including its uses side effects and safety interactions pictures warnings and user ratings. Xyosted autoinjector testosterone enanthate.
Using hormones to change physical characteristics -AND- b. It is not known if Xyosted is safe and effective in children younger than 18 years old. Food and Drug Administration FDA announced the approval of Xyosted.
Xyosted is a prescription medicine that contains testosterone. Selling or giving away this medicine may harm others and it. Xyosted autoinjector testosterone enanthate.
Xyosted is used to treat adult men who have low or no testosterone due to certain medical conditions. This medication can raise your blood pressure which can increase your risk of having a heart attack or stroke.